Synergistic Action of Commercial Lipase, Lipoxygenases and Proteinase towards PLA Depolymerization
Synergistic Action of Commercial Lipase, Lipoxygenases and Proteinase towards PLA Depolymerization
Salini, A.; Gonnelli, P.; Padoan, C.; Helali, Y.; Waeytens, J.; Fusco, S.; Cannella, D.
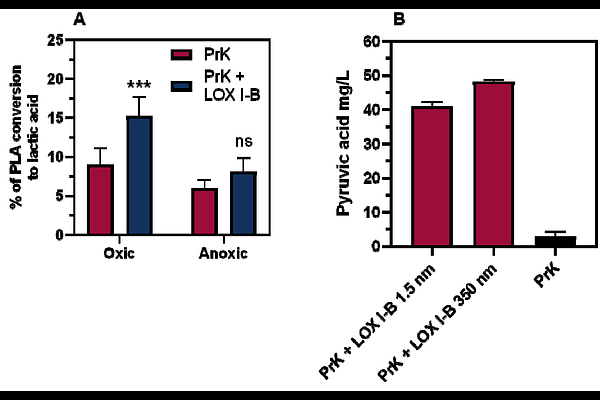
AbstractPolylactic acid (PLA) is a biobased aliphatic polyester produced from renewable resources. Its building block is lactic acid (LA), which is mainly produced through fermentation processes rather than chemical synthesis, as fermentation enables the production of optically pure D-LA or L-LA, instead of a racemic mixture. In this work, three commercial enzymes Proteinase K (PrK) from Tritirachium album, Candida rugosa lipase (CrLip), and Glycine max lipoxygenase (LOX I-B) were investigated for their synergistic action for the degradation of solvent-cast as well as EoL-PLA films. While Lip and LOX I-B alone did not promote PLA hydrolysis, they significantly enhanced PrK-mediated hydrolysis by 5.7-fold and 2.5-fold, respectively. Lip, and to a lesser extent LOX I-B, increased PrKs productive binding to the polymer, thus accelerating catalysis. These findings underline the potential of synergistic enzymes for PLA degradation and provide the first evidence of lipoxygenase activity in this process.