Emergent higher-order interactions enable coexistence in phage-bacteria community dynamics
Emergent higher-order interactions enable coexistence in phage-bacteria community dynamics
Dey, R.; Coenen, A. R.; Solonenko, N. E.; Burris, M. N.; Mackey, A. I.; Galasso, J.; Sun, C. L.; Demory, D.; Muratore, D.; Beckett, S. J.; Sullivan, M. B.; Weitz, J. S.
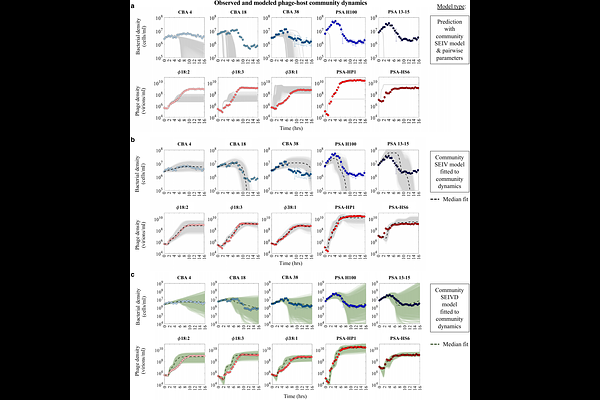
AbstractDiverse phage-bacteria communities coexist at high densities in environmental, agricultural, and human-associated microbiomes. Phage-bacteria coexistence is often attributed to coevolutionary processes mediated by complex, pairwise infection networks. Here, using in vitro experiments and mathematical models, we explore how higher-order interactions function as a complementary, ecological feedback mechanism to stabilize phage-bacteria communities. To do so, we examine an environmentally-derived, synthetic phage-bacteria community comprised of five marine heterotrophic bacteria (Cellulophaga baltica and Pseudoalteromonas strains) and five associated phage. We used Bayesian inference to reconstruct free phage production in one-step growth experiments and then forecasted pairwise phage-bacteria dynamics over multiple infection cycles. In contrast to model predictions of rapid bacterial population collapse, each bacterial strain persisted, consistent with density-dependent stabilization mechanisms at high virus densities. We then extended models into a community context and found an additional stabilization mechanism: life history traits differ in community vs. pairwise contexts. Follow-up experiments confirm that lysis is inhibited at relatively high viral densities and phage traits (including burst size) can shift when infecting single vs. multiple strains. More broadly, these findings suggest that complex community coexistence of phage and bacteria may be more common than anticipated when including feedback mechanisms outside of regimes of fitted pairwise models that do not reflect the full scope of ecologically relevant contexts.