Effects of priority on strain-level composition of the honey bee gut community
Effects of priority on strain-level composition of the honey bee gut community
Jones, K. R.; Song, Y.; Rinaldi, S. S.; Moran, N. A.
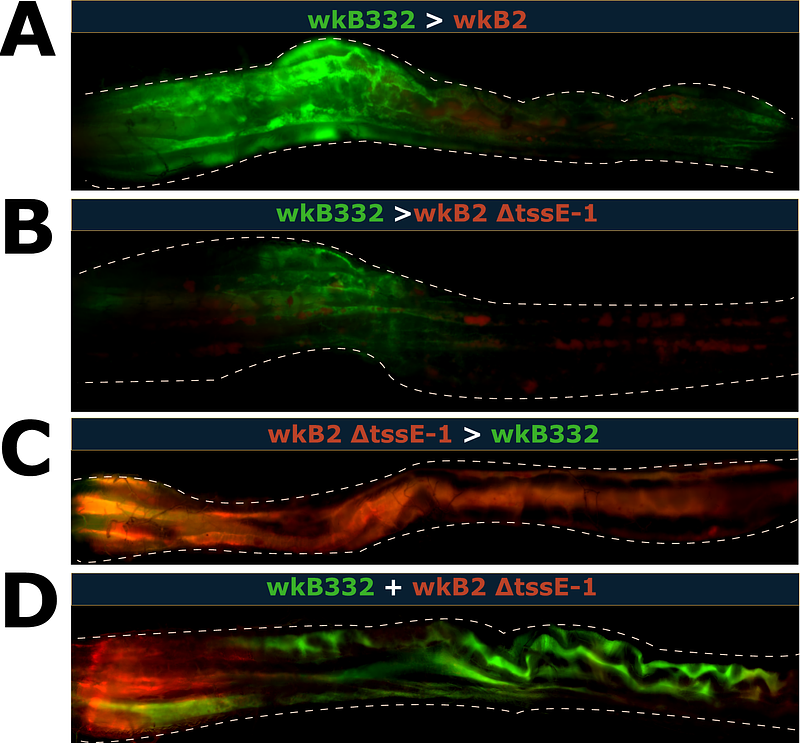
AbstractHost-associated microbiomes are complex communities shaped by interactions between members. The Type VI Secretion System (T6SS), among other bacterial weapons, allows gram-negative bacteria to deliver toxic effectors into competitors. In this study, we investigated the impact of differential colonization timing on the competitive advantage associated with T6SS possession using Snodgrassella alvi, a core symbiont of the honey bee gut microbiome. Following a timeline based on the natural establishment window of the gut microbiome, we sequentially inoculated newly emerged bees with fluorescently labeled strains that differed in presence of the T6SS-1. When inoculated simultaneously, the T6SS-1-possessing strain (wkB2) consistently excluded the T6SS-1-lacking strain (wkB332); however, when given a five-day advantage, the second strain was consistently excluded regardless of strain identities. With a one-day advantage, the effect of priority was weakened, but wkB332 was able to persist following introduction of wkB2. Utilizing a wkB2 T6SS-1 knockout strain, we repeated our 24-hour priority experiments and found that the T6SS-1 contributes to invasion outcomes along with other mechanisms of competition. Through fluorescent microscopy, we explored how coexisting strains in these experimental scenarios organize spatially within the bee ileum. Our results demonstrate that colonization timing can have lasting consequences for strain composition of the established microbiome. These findings illustrate the influence of stochastic processes in microbial community assembly and emphasize that differences in colonization timing may alter competitive outcomes between taxa, impacting taxon coexistence.