A continuum reaction-diffusion model for spread of gene silencing in chromosomal inactivation
A continuum reaction-diffusion model for spread of gene silencing in chromosomal inactivation
Paul, S.; Sun, S.; Haynes, K.; Hong, T.
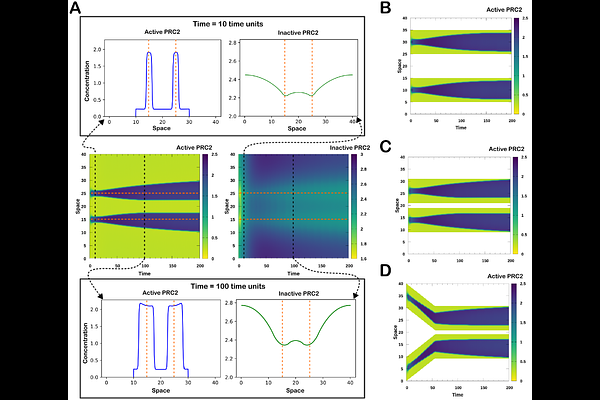
AbstractRegulation of gene silencing in large regions of chromosomes is crucial for development and disease progression, and there has been an increasing interest in using it for new therapeutics. One example of massive gene silencing is X chromosome inactivation (XCI), a process essential for dosage compensation of X-linked genes. During XCI, most genes in the X chromosome are inactivated following the transcription of XIST, an X-linked long noncoding RNA. Recent experiments with transgenes showed that the spread of gene silencing can be induced by XIST transcription in cis, but the spread is restricted in space. The mechanism of controlling the spread remains unclear. In this work, we develop a continuum reaction-diffusion model that elucidates chromosomal inactivation through a bistable system governed by a regulatory network for XIST-mediated gene silencing. We find that the spread of XIST can be tuned by known negative feedback loops regulating its synthesis and degradation, and that the spread of gene silencing is controlled by a wave-pinning mechanism in which both global regulation of silencing complex and local variations of histone modifications can play crucial roles. In addition, we integrate the discrete three-dimensional arrangement of the X chromosome and autosomes into this continuous model. We use a 3D chromosome structure inferred from experimental data and our modeling framework to show the spatiotemporal regulation for spread of gene silencing. Our method enables the investigation for the inactivation dynamics of large regions of chromosomes with varying degrees of the spread of gene silencing. Our model provides mechanistic insights that quantitatively relate gene regulatory networks to tunability and stability of chromosomal inactivation.