Exploring Protein Patterns, Cavity Interactions, and Therapeutic Insights in Cancer
Exploring Protein Patterns, Cavity Interactions, and Therapeutic Insights in Cancer
Tejera Nevado, P.; Otero Carrasco, B.; Rodriguez Gonzalez, A.
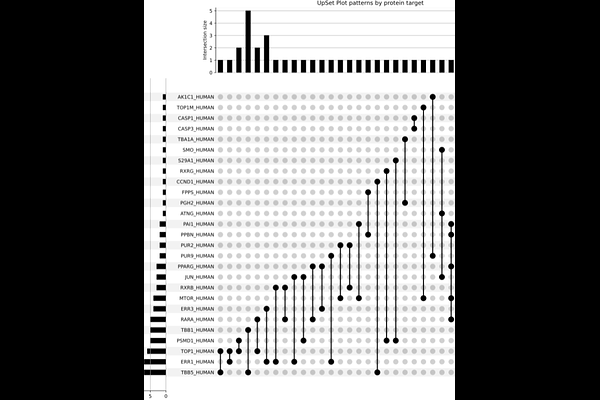
AbstractProtein sequence alignments are essential for identifying proteins\' shared structural and functional features. Detecting short amino acid sequences, termed patterns, across lung cancer and other related datasets facilitates the identification of relevant features. This study builds on previous findings by exploring proteins that share common patterns already identified. Using sequence matching at 5% and 10% occurrence thresholds, we identified 2,368 and 47 patterns, respectively. To reduce complexity and refine the dataset, shorter patterns from the 10% occurrence streamlined the analysis by isolating highly relevant patterns while reducing redundancy among proteins sharing sequence segments. Subsequent analyses integrated structural predictions for protein folding comparison, enabling the detection of patterns in different proteins and the identification of potential key residues. During cavity detection prediction, some amino acids were inspected in detail to assess their impact on protein function and their relevance in drug-target interactions. These insights were considered during docking studies, focusing on proteins used in treatments with pre-described ligands. By connecting raw sequence data to folding structures and functional features, we identified critical protein cavities that underscore the role of mutations in altering protein behavior and influencing drug-target interactions. These findings highlight protein activity\'s structural foundations and their importance in understanding cancer biology. By uncovering conserved sequence patterns and their structural implications, this study provides insights into potential biomarkers and therapeutic targets, that could aid in developing more effective cancer treatments.