The 1p/19q co-deletion induces targetable and imageable vulnerabilities in glucose metabolism in oligodendrogliomas
The 1p/19q co-deletion induces targetable and imageable vulnerabilities in glucose metabolism in oligodendrogliomas
Udutha, S.; Batsios, G.; Taglang, C.; Gillespie, A. M.; Viswanath, P.
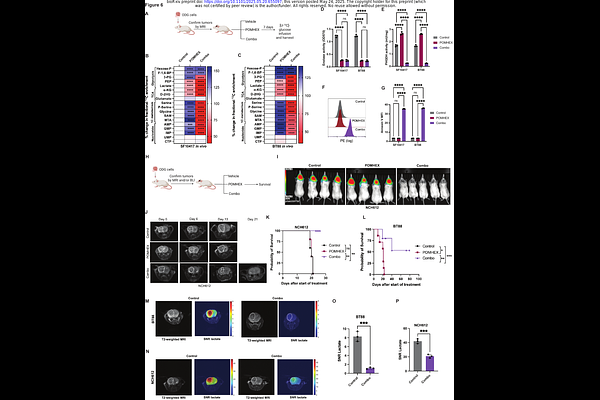
AbstractBackground: The 1p/19q co-deletion is a hallmark of oligodendrogliomas. The goal of this study was to exploit metabolic vulnerabilities induced by the 1p/19q co-deletion for oligodendroglioma therapy and non-invasive imaging. Methods: We used stable isotope tracing, mass spectrometry, and genetic and pharmacological approaches to interrogate [U-13C]-glucose metabolism in patient-derived oligodendroglioma models (SF10417, BT88, BT54, TS603, NCH612). We examined whether tracing [6,6-2H]-glucose metabolism using deuterium metabolic imaging (DMI) provided an early readout of treatment response. Results: The expression of the glycolytic enzyme enolase 1 (ENO1; chromosome 1p36.23) was reduced in patient-derived oligodendroglioma cells and patient biopsies due to the 1p/19q co-deletion and histone hypermethylation. Conversely, ENO2 was upregulated, an effect that was driven by mitogen-activated protein kinase (MAPK) signaling and ERK1-mediated phosphorylation and inactivation of the CIC transcriptional repressor in oligodendrogliomas. Genetic ablation of ENO2 or pharmacological inhibition using POMHEX inhibited proliferation with nanomolar potency but was not cytotoxic to oligodendroglioma cells or tumor xenografts. Mechanistically, ENO2 loss abrogated [U-13C]-glucose metabolism to lactate but shunted glucose towards biosynthesis of serine and purine nucleotides, an effect that was driven by phosphoglycerate dehydrogenase (PHGDH). Importantly, the PHGDH inhibitor D8 was synthetically lethal in combination with POMHEX, and the combination induced tumor regression in vivo. Furthermore, DMI of lactate production from [6,6-2H]-glucose provided an early readout of response to combination therapy that preceded MRI-detectable alterations and reflected extended survival. Conclusions: We have identified ENO2 and PHGDH as 1p/19q co-deletion-induced metabolic vulnerabilities in oligodendrogliomas and demonstrated that DMI reports on early response to therapy.