An improved model for prediction of de novo designed proteins with diverse geometries
An improved model for prediction of de novo designed proteins with diverse geometries
Orr, B.; Crilly, S. E.; Akpinaroglu, D.; Zhu, E.; Keiser, M. J.; Kortemme, T.
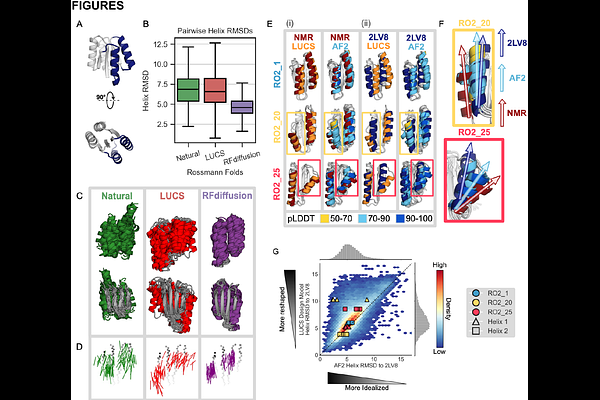
AbstractNature uses structural variations on protein folds to fine-tune the geometries of proteins for diverse functions, yet deep learning-based de novo protein design methods generate highly regular, idealized protein fold geometries that fail to capture natural diversity. Here, using physics-based design methods, we generated and experimentally validated a dataset of 5,996 stable, de novo designed proteins with diverse non-ideal geometries. We show that deep learning-based structure prediction methods applied to this set have a systematic bias towards idealized geometries. To address this problem, we present a fine-tuned version of Alphafold2 that is capable of recapitulating geometric diversity and generalizes to a new dataset of thousands of geometrically diverse de novo proteins from 5 fold families unseen in fine-tuning. Our results suggest that current deep learning-based structure prediction methods do not capture some of the physics that underlie the specific conformational preferences of proteins designed de novo and observed in nature. Ultimately, approaches such as ours and further informative datasets should lead to improved models that reflect more of the physical principles of atomic packing and hydrogen bonding interactions and enable improved generalization to more challenging design problems.