Magnesium-28: A Novel Self-Theranostic Approach through Targeting Metabolic Enzyme Disruption and Intracellular Radiation.
Magnesium-28: A Novel Self-Theranostic Approach through Targeting Metabolic Enzyme Disruption and Intracellular Radiation.
Van Luyen, T.
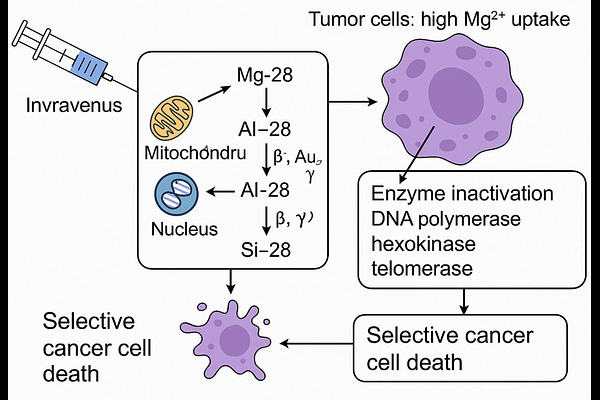
AbstractThe limitations of conventional cancer therapies, such as low selectivity and significant side effects, necessitate innovative approaches. This study proposes a pioneering self-theranostic strategy using Magnesium-28 (Mg-28) alone, enabling simultaneous diagnosis, therapy, and therapy control. Exploiting the elevated Mg2+ demand in cancer cells, Mg-28 self-targets Mg-dependent enzymes (e.g., DNA/RNA polymerases, hexokinase, telomerase) within intracellular organelles like the nucleus and mitochondria, without bio-chemical carriers or nanoparticles as recently methods. A theoretical model, based on the Mg-uptake coefficient, predicts selective Mg-28 accumulation in tumors following intravenous administration. The Mg-28 chain decays into Aluminum-28 then Silicon-28, delivering highly localized irradiation via beta particles, Auger electrons, and recoil ions to critical intracellular structures, while disrupting essential Mg-dependent enzymes for a dual mechanism of radiotherapy and multi-enzyme inactivation. Simulations of Linear Energy Transfer (LET), radiation range, and absorbed dose show that nanogram-scale amounts of Mg-28 can deliver 60-400 Gy to tumors ranging from 0.03 mg to 500 g in size, suggesting potential cytotoxicity that could be effective across not only a broad range of stages but also types of cancer due to the fundamental role of magnesium in cancer cell metabolism and proliferation. Mg-28 and its daughter\'s gamma emissions support early tumor detection and real-time treatment monitoring, enhancing precision. As the first proposed single-isotope theranostic approach leveraging magnesium dependency, this innovative strategy provides a robust foundation for future preclinical and clinical investigations aimed at validating its therapeutic efficacy, pharmacokinetics, and biosafety inaugurating a novel hypothesis for cancer therapy.