Modeling the Co-Activation of Inflammatory Genes1Mediated by NFκB and IRF-3 During Viral Infections
Modeling the Co-Activation of Inflammatory Genes1Mediated by NFκB and IRF-3 During Viral Infections
Tiwari, S.; Basak, S.; Pandey, R.
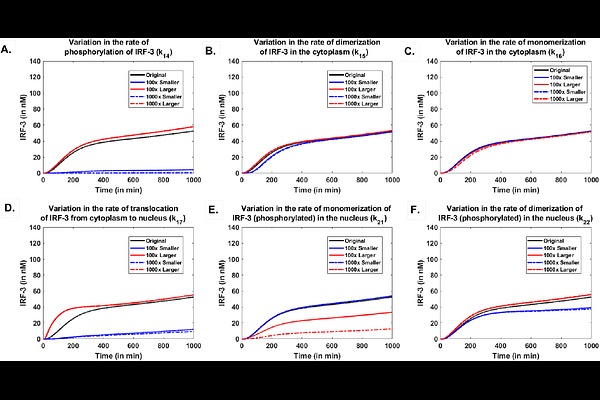
AbstractInnate immune responses of host cells are activated upon exposure to external stimuli such as viral infections. These activations trigger the signaling pathways involved in antiviral responses. Activated pathways play a crucial role in the regulation of inflammatory and antiviral responses involving transcription factors, cytokines, cell surface viral sensors, and the activation of target genes. Although the role of key transcription factors such as NFkB and IRF-3 in the regulation of antiviral genes that encode type I interferons (IFNalpha/beta) is identified and well explored. The precise mechanistic understanding that governs their complex context-dependent temporal activation remains elusive. Here, we have developed a mathematical model to demonstrate the co-regulation of the expression of the IFN$\\beta$ gene by Nuclear Factor kappa B (NFkB) and IFN Regulatory Factor-3 (IRF-3) after their activation in host cells by the viral analogue Poly I:C. In our model, the binding activities of the transcription factors NFkB and IRF-3 at the IFNbeta promoter are modeled using a probability-based framework. Our results demonstrate that the expression pattern of IFNbeta post-viral infections would be pertaining to the co-activation of that by NFkB and IRF-3. In addition, the immediate expression of the IFN$\\beta$ gene and, thus, the initial host response would be mainly attributed to immediate activation of NFkB while IRF-3 would be responsible for delayed but persistent expression of the IFNbeta. Our model provides an underlying molecular mechanism for a similar response observed for Newcastle disease virus and the Vesicular stomatitis virus infection. Furthermore, we suggest that the rapid as well as an elevated and persistent expression of IFNbeta, would be observed later due to a temporal bias in binding of both transcription factors NFkB and IRF-3, highlighting the need for coregulation of IFNbeta by two different signaling pathways in a sequential manner. Also, our model predicts that the binding affinity of IRF3 to the promoter region of IFNbeta would be equal to or larger than that of NFkB for a strong and persistent antiviral responses.