Discovery and structure-activity relationship analysis of 2-Pyridyl Thienopyrimidine derivatives as promising therapeutic candidates for the treatment of Buruli ulcer
Discovery and structure-activity relationship analysis of 2-Pyridyl Thienopyrimidine derivatives as promising therapeutic candidates for the treatment of Buruli ulcer
Keumoe, R.; Kouipou, R. M. T.; Madiesse, E. A. K.; Fokou, J. B. H.; Dize, D.; Fokou, P. V. T.; Kamdem, B. P.; Makembe, A. H. E.; Njionhou, M. S. N.; Ghoshal, A.; Kundu, A.; Laleu, B.; Boyom, F. F.
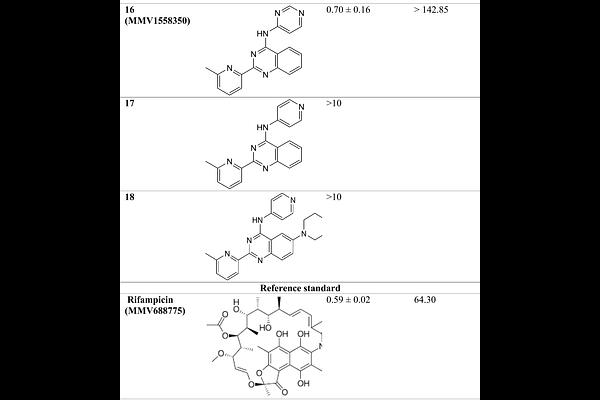
AbstractMycobacterium ulcerans, the bacterium causing Buruli ulcer (BU), can potentially develop resistance to existing antibiotics (rifampicin-clarithromycin/moxifloxacin), underscoring the need for new antimycobacterial treatments. This study screened the Pathogen Box from Medicines for Malaria Venture (MMV) to identify M. ulcerans inhibitors. Four hit compounds were found, including the 2-(6-methylpyridin-2-yl)-N-(pyrimidin-4-yl)thieno[3,2-d]pyrimidin-4-amine MMV688122 as a novel anti-M. ulcerans chemotype. Synthesis of structural analogues of MMV688122 allowed the identification of 2-(4-methylpyridin-2-yl)-N-(pyrimidin-4-yl)thieno[3,2-d]pyrimidin-4-amine MMV1578877 as the most potent, with submicromolar activity. Importantly, this analogue was non-cytotoxic up to 100 {micro}M in human fibroblasts. Structure-activity relationship (SAR) studies indicated the crucial role of the methylpyridin-2-yl group in inhibiting M. ulcerans and the possibility to replace the thienopyrimidine core by a quinazoline. While MMV1578877 showed better metabolic stability than MMV688122, further improvement and testing in real-world M. ulcerans clinical isolates are still required. Further metabolite identification and SAR data should guide the optimization of this novel chemotype to enable in vivo testing.