Loss of UFMylation supports prostate cancer metastasis and rewires cell metabolism towards hexosamine biosynthesis
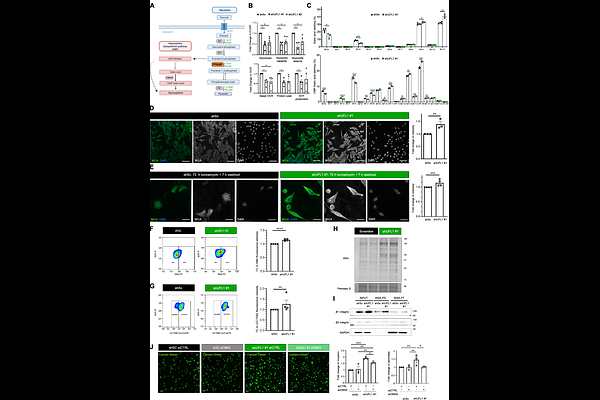
Loss of UFMylation supports prostate cancer metastasis and rewires cell metabolism towards hexosamine biosynthesis
Bozal Basterra, L.; Salazar Palacio, M. C.; Ferreira Campos, A. M.; Demicco, M.; Schmidt, D. R.; Sobczak, K.; Ereno-Orbea, J.; Altea Manzano, P.; Miranda Villanueva, A.; Martinez La Osa, B.; Garcia-Longarte, S.; Ponce Rodriguez, M.; Mendizabal, I.; Carlevaris Lopez, O.; Astobiza, I.; Martin Martin, N.; Zabala-Letona, A.; Talamillo, A.; Fernandez Garcia, J.; Azkargorta, M.; Iloro, I.; Barrio, R.; Elortza, F.; Fendt, S. M.; Vander Heiden, M. G.; Jimenez Barbero, J.; D. Sutherland, J.; Carracedo, A.
AbstractThe acquisition of metastatic features in tumor cells encompasses genetic and non-genetic adaptation, including reprogramming of cellular metabolism. Here we show that loss of UFMylation reroutes glucose metabolism, promotes invasive capacity and supports prostate cancer metastasis. Through transcriptome-based bioinformatics analysis, we identified a reduction in the ubiquitin-like modifier UFM1 and its ligase UFL1 in metastatic prostate cancer. We demonstrate that loss of UFMylation results in enhanced cancer cell dissemination and a switch from cellular proliferation to invasion. Using biotin-based proteomics, we identified phosphofructokinase (PFKAP) as an unprecedented UFMylation substrate. Consistent with UFMylation playing a role in the regulation of phosphofructokinase activity, loss of UFMylation reduced glucose metabolism in favour of hexosamine biosynthesis, which resulted in elevated glycosylation of proteins relevant for cell invasion. These results reveal a role for UFMylation in the regulation of phosphofructokinase and glucose metabolism to support prostate cancer metastasis.