Local, but not circulating, complement C3 governs immune checkpoint blockade efficacy
Local, but not circulating, complement C3 governs immune checkpoint blockade efficacy
Miyai, Y.; Shiraki, Y.; Sugiyama, D.; Sato, Y.; Kato, K.; Asai, N.; Cao, F.; Nagao, N.; Tanabe, K.; Nakatochi, M.; Hase, T.; Yoshikawa, T. F. C.; Kobayashi, T.; Iwama, S.; Esaki, N.; Mii, S.; Arima, H.; Kimura, H.; Takahashi, M.; Ando, Y.; Enomoto, A.
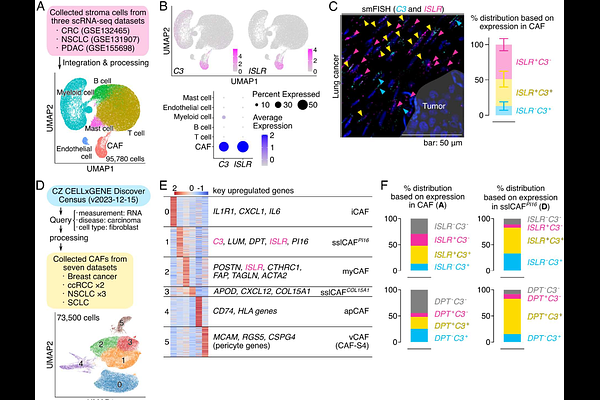
AbstractThe roles of circulating complement C3, produced in hepatocytes, in immune defense are well defined in the literature. Interestingly, the C3 gene evolved before the circulatory system, suggesting an essential but yet unknown role of locally produced C3. Here, we investigated the effect of C3 on cancer immunotherapy and found that cancer-associated fibroblast (CAF)-derived C3, not hepatocyte-derived C3, determines the efficacy of immune checkpoint blockade (ICB). Tumors developed in CAF-specific C3 knockout mice exhibited resistance to anti-PD-1 therapy, with increased immunosuppressive M2-like macrophage infiltration. Mechanistically, the C3 degradation product iC3b suppressed myeloid cell infiltration via complement receptor 3 signaling, and the therapeutic targeting of this pathway restored ICB sensitivity of immunotherapy-resistant tumors. In human cancers, stromal C3 expression correlates with reduced M2-like macrophage infiltration and improved immunotherapeutic outcomes. These data demonstrate the role of locally produced C3 in the innate immune response, which may have been conserved across many organisms.