Integrative Multi-Omics Analysis Identifies Nuclear Factor I as a Key Driver of Dysregulated Purine Metabolism in DIPG
Integrative Multi-Omics Analysis Identifies Nuclear Factor I as a Key Driver of Dysregulated Purine Metabolism in DIPG
Mersich, I.; Congrove, S.; Horchar, M.; Caceres, R.; Muraleedharan, R.; Desai, J.; Desai, P.; Sallans, L.; Reisz, J. R.; Grier, A.; Hass, M.; Donmez, O.; Yin, C.; Weirauch, M. T.; Kottyan, L. T.; Stevenson, C.; Sun, C.; deBlank, P.; Pillay-Smiley, N.; Hummel, T.; Elumalai, N.; Tavassoli, A.; Firestein, R.; Phoenix, T.; D'Alessandro, A.; Dasgupta, B.
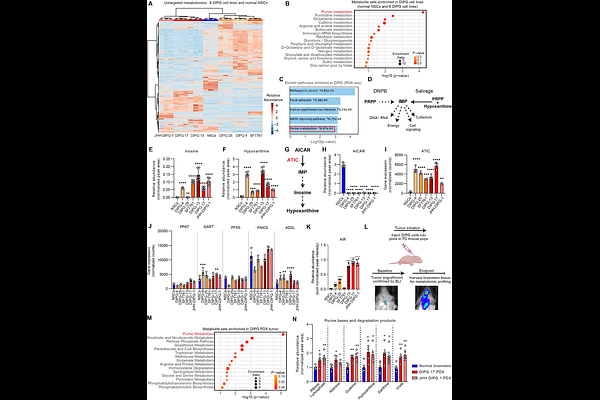
AbstractDiffuse intrinsic pontine glioma (DIPG) is a devastating brainstem cancer in children, with a median survival of under one year and limited treatment options. Over 80% of DIPGs possess a H3K27M mutation. To identify metabolic vulnerabilities linked to this mutation, we utilized a multi-omics approach in H3K27M-expressing cells, patient-derived cell lines, and mouse models. We show that by reprogramming chromatin landscape the mutation aberrantly induces NFI transcriptional activity, leading to misregulated purine metabolism. The mutation amplifies purine biosynthesis and degradation via the enzymes ATIC and PNP, respectively. Unregulated purine degradation relieves the negative feedback of purines on their own synthesis allowing continuous synthesis, use and degradation making DIPGs reliant on purine biosynthesis. Targeting ATIC reduced tumor progression and improved survival in mice. We propose ATIC as a potential novel target in DIPG.