Structural insights into context-specific inhibition of bacterial translation by macrolides
Structural insights into context-specific inhibition of bacterial translation by macrolides
Syroegin, E. A.; Aleksandrova, E. V.; Kruglov, A. A.; Paranjpe, M. N.; Svetlov, M. S.; Polikanov, Y. S.
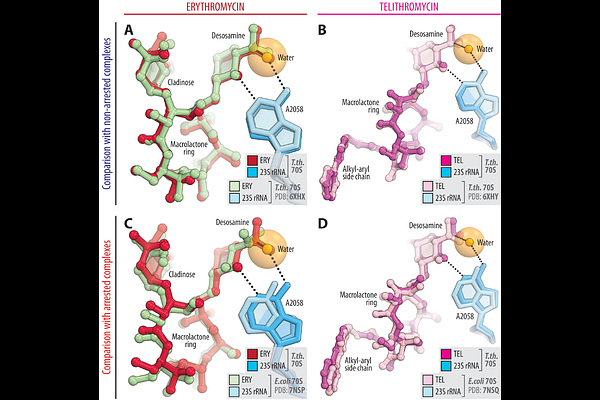
AbstractThe ribosome\'s peptidyl transferase center (PTC) catalyzes peptide bond formation during protein synthesis and is targeted by many antibiotic classes. Remarkably, macrolides that bind in the peptide exit tunnel some ~10A away from the PTC also remotely inhibit PTC and cause translational arrest depending on the synthesized polypeptide sequence. The Arg/Lys-X-Arg/Lys (also known as +X+) motif is particularly susceptible to this inhibition, as peptidyl-tRNA carrying nascent peptide with penultimate arginine or lysine residue fails to react with aminoacyl-tRNA carrying the same amino acids in the presence of macrolides. While structural studies of macrolide-bound ribosomes have shed light on the context-specific nature of this inhibition, the precise roles of the drug, ribosome, and tRNA in modulating PTC activity remain unclear. In this study, we present a detailed structural analysis of ribosome-nascent chain complexes (RNCs) that represent either arrested or non-arrested states, containing various combinations of peptidyl- and aminoacyl-tRNAs, with or without macrolides. Our findings reveal a dynamic interaction between the ribosome-bound drug, the nascent peptide, and the incoming amino acid, which collectively modulates PTC function. This lays the foundation for designing antibiotics that can overcome drug resistance by preventing the induction of inducible erm genes in pathogens.