A New Class of Precision Therapeutics that Inhibit Prostate Cancer Mediated Bone Destruction
A New Class of Precision Therapeutics that Inhibit Prostate Cancer Mediated Bone Destruction
Qiao, F.; Gordon, R.; Pattanayak, A.; Li, W.; Knibbe-Hollinger, J.; O'Neill, K.; Chen, W.; Mukthapuram, P. R.; Natarajan, A.; Bergan, R.
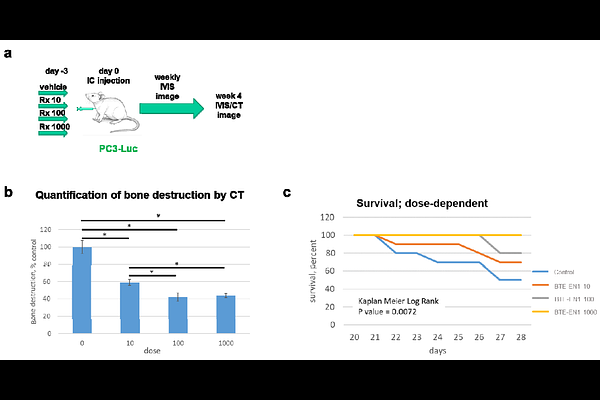
AbstractCancer mediated bone destruction remains a common cause of morbidity and mortality. Here we couple an inhibitor of cell movement to bone trophic bisphosphonates, forming heterobifunctional compounds termed Dual Acting Bone Defender agents (DABDs). DABDs inhibit prostate cancer cell motility and osteoclast mediated bone destruction in vitro. Our lead compound, BTE-EN1, inhibits circulating human prostate cancer cells from forming bone destructive lesions in mice and prolongs life in a dose-dependent fashion. In mice with established metastatic lesions, BTE-EN1 significantly decreases bone destruction by over 60% compared to the control. Mechanistically, BTE-EN1 binds hydroxyapatite, inhibits prenylation of Rap1A, induces apoptosis in osteoclasts, and inhibits Raf1 activation and formation of bone resorptive cavities in osteoclasts, thus retaining the function of its constituent chemicals. BTE-EN1 is not toxic to mice at doses 4,000-fold over those required for efficacy, exhibits anti-cell motility activity across other bone-destructive cancer cell types, and does not interfere with standard-of-care hormone therapy or chemotherapy. BTE-EN1 is a new class of highly active therapeutics that inhibit cancer mediated bone destruction and holds high potential for translation into humans.