A Precision Gene Engineered B Cell Medicine Producing Sustained Levels of Active Factor IX for Hemophilia B Therapy
A Precision Gene Engineered B Cell Medicine Producing Sustained Levels of Active Factor IX for Hemophilia B Therapy
Liu, H.; Morgan, R. A.
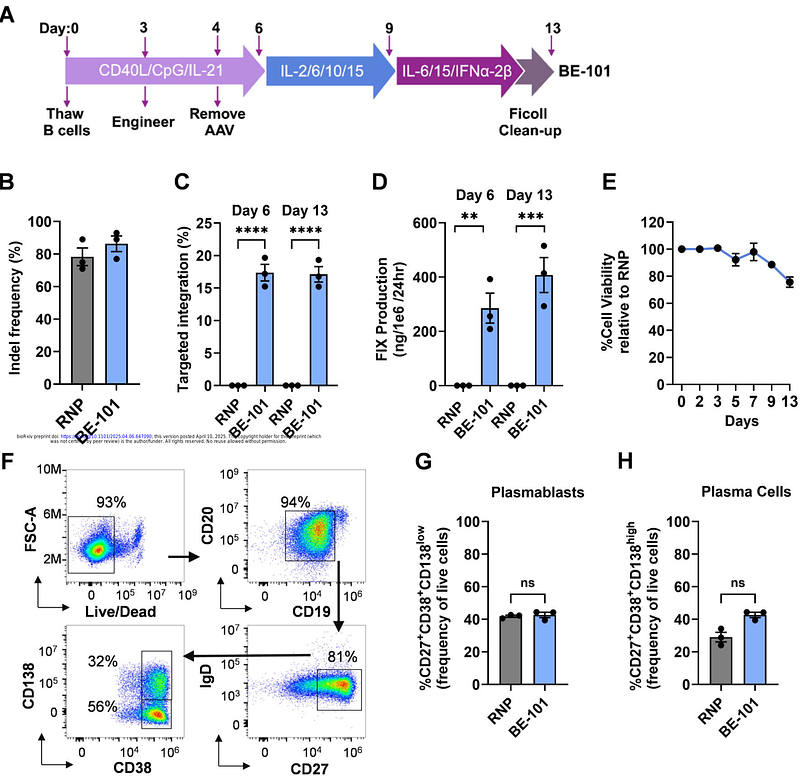
AbstractHemophilia B gene therapy treatments currently have not addressed the need for predictable, durable, active, and redosable factor IX (FIX). Unlike conventional gene therapy, engineered B Cell Medicines (BCMs) are durable, redosable, and titratable, and thus have the potential to address significant unmet needs in the Hemophilia B treatment paradigm. BE-101 is an autologous BCM comprised of expanded and differentiated B lymphocyte lineage cells genetically engineered ex vivo to secrete FIX-Padua. CRISPR/Cas9 mediated gene editing at the C-C chemokine receptor type 5 locus was used to facilitate transgene insertion of an AAV6-encoded DNA template via homology-directed repair. Transgene insertion did not alter B cell biology, viability, or differentiation into plasma cells. Appreciable levels of BE-101-derived FIX-Padua were detected within 1 day after IV administration in mouse and steady state was reached within 2 weeks and persisted for over 184 days. Redosing produced an increase in FIX-Padua production close to linear dose proportionality. Comprehensive genotoxicity analysis found no off-target issues of concern. No safety signals were observed in animal tolerability and GLP toxicology studies. In conclusion, BE-101 produces sustained levels of active FIX-Padua with the ability to engraft without host preconditioning and with the potential for redosing and titratability.