FAM83H regulates postnatal T cell development through thymic stroma organization
FAM83H regulates postnatal T cell development through thymic stroma organization
Ogan, B. M.; Forstlova, V.; Dowling, L. J.; Simova, M.; Vicikova, K.; Novosadova, V.; Spoutil, F.; Cervenkova, S.; Prochazkova, M.; Tureckova, J.; Fedosieieva, O.; Labaj, J.; Nickl, P.; Krizova, K.; Prochazka, J.; Sedlacek, R.; Balounova, J.
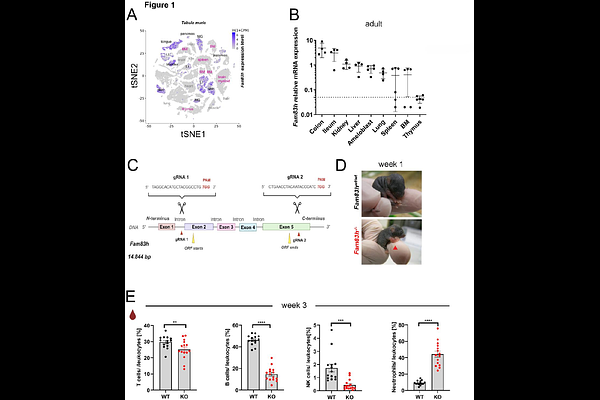
AbstractFamily of Sequence Similarity 83H (FAM83H/ SACK1H) is primarily expressed in epithelial cells, where it interacts with casein kinase 1 (CK1) and keratins to regulate cytoskeletal organization, cell proliferation, and vesicular trafficking. Mutations in FAM83H are known to cause amelogenesis imperfecta, highlighting its critical role in enamel formation. We generated Fam83h-deficient mice (Fam83hem2(IMPC)Ccpcz, Fam83h-/-) and mice lacking a part of the N-terminal CK1-binding domain (Fam83h{triangleup}87/{triangleup}87). Consistent with other Fam83h-deficient models, these mice are subviable, smaller in size, and exhibit a sparse, scruffy coat, scaly skin, general weakness, and hypoactivity. Notably, both strains show impaired lymphoid cell development in early postnatal life. In the thymus, Fam83h expression is confined to thymic epithelial cells (TECs), and its deficiency in stromal cells results in disrupted thymic architecture and severe block in the expansion of DN3 (double-negative stage 3) T cells, ultimately leading to insufficient T cell production. Single-cell transcriptomic analysis reveals that Fam83h-/- cortical TECs (cTECs) express reduced levels of the TEC master regulator Foxn1, and several of its downstream target genes, suggesting a critical role for FAM83H and CK1 in cTEC maturation.