Dynamic estimation of metabolic state during CAR T cell production and the relationship of early metabolism to final therapeutic product
Dynamic estimation of metabolic state during CAR T cell production and the relationship of early metabolism to final therapeutic product
Jagannathan, N. S.; Sin, W.-X.; Teo, D. B. L.; Kairi, F.; Luah, Y. H.; Lim, F. L. W. I.; Seng, M. S.-F.; Soh, S. Y.; Lee, Y. H.; Tucker-Kellogg, L.; Birnbaum, M. E.; Ram, R. J.
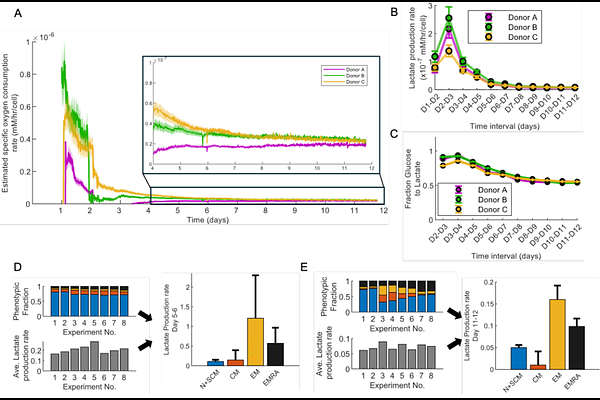
AbstractAdoptive cell therapies such as CAR T cells have revolutionized cancer treatment and shown successes even with refractory cases of haematological malignancies. There is burgeoning interest in the optimization and improved manufacturing of cell therapy products. For CAR T cell therapy, enrichment of certain phenotypes of T cells in the infusion product have been correlated with improved long-term treatment outcomes. While metabolic control of T cell phenotypic fates has been demonstrated in some contexts, gaps still exist in our knowledge of how T cell metabolic dynamics early during CAR T manufacturing might affect critical quality attributes (CQAs) of the final product (e.g., differentiation/exhaustion/potency). We present a modelling framework that can perform real-time estimation of per-cell metabolic rates of T cells expanded ex vivo in a reactor. We validate our estimated rates using metabolic assays, show how average rates can be deconvoluted to rates of individual T cell phenotypes, and demonstrate applicability to different reactor types. Applying our tool to the expansion of both healthy and patient-derived cells in a perfusion-based microbioreactor, we offer proof-of-principle to show that correlations exist between early metabolic rates of T cells in culture and cellular attributes related to growth, differentiation and exhaustion of the final product. Given the biological variation that exists in the growth and dynamics of patient-derived cells in culture, such modelling contributes to the overarching goal of improving the consistency of cell therapy through Adaptive Process Control (APC).